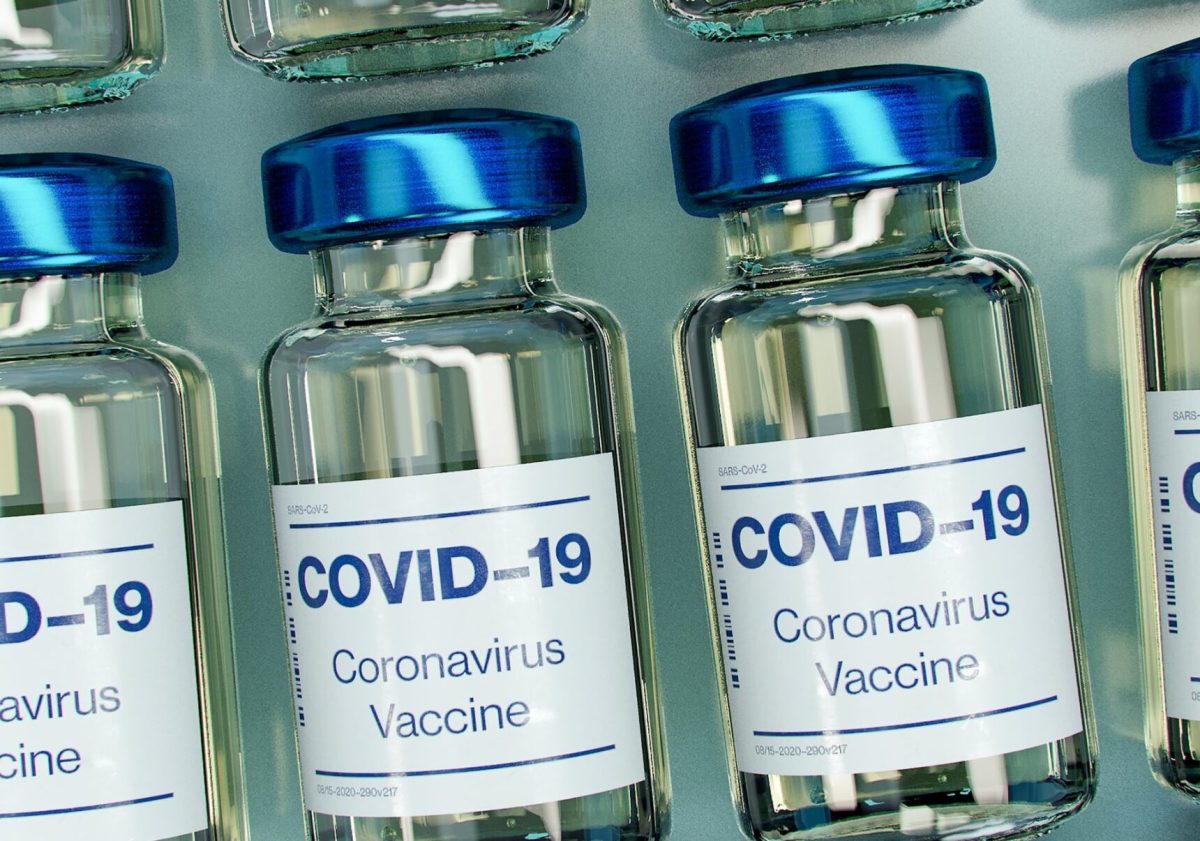
While the COVID-19 pandemic has swept across the world, threatening and devastating the lives of millions, governments and multinational corporations have been racing to find a vaccine. The virus, afflicting vulnerable groups such as those 65 and older, with compromised immune systems, or economically disadvantaged, continues to rapidly spread as experts warn the holiday season will only worsen conditions.
Currently, Russia and China have approved a total of six vaccines, all of which were approved without waiting for the results of the Phase 3 clinical trials. According to the Center for Disease Control and Prevention (CDC), Phase 3 trials involve the testing of hundreds or thousands of participants. While not required in Russia or China, this testing can be key in determining common side effects as well as the overall safety of a vaccine that could be distributed to an entire country. A vaccine only receives the FDA’s (U.S. Food and Drug Administration) seal of approval if the trials and studies, in comparison with the method of manufacture, prove that the benefits outweigh the potential risks posed by the distribution of the vaccine. Globally, there are 13 vaccines that are actively in their Phase 3 trial, three of which are from the U.S.
On May 15, 2020, Operation Warp Speed (OWS) was launched by the U.S. government to fasttrack the development, production and distribution of a potential COVID-19 vaccine. OWS has provided funding to over 14 vaccine candidates, and three of the 14 vaccines have advanced to their Phase 3 trial. These three vaccines are from the pharmaceutical giants Pfizer, Moderna and AstraZeneca.
Not officially part of OWS until July 2020, Pfizer is an American multinational pharmaceutical company that invested $2 billion into developing a vaccine. On Nov. 10, 2020, Pfizer, in partnership with the German company BioNTech, announced that its vaccine candidate has shown a 90% effectiveness against COVID-19. With the announcement of this news, the stock market celebrated with a 1.2% increase in the S&P 500 and a 800 point rise in the Dow Jones Industrial Average. The results were based on Pfizer’s Phase 3 trial, which officially concluded on Nov. 18, 2020. While it could be months before the vaccine is distributed to millions of people, this news comes as a sign of hope in a crisis that is close to reaching its one-year anniversary. As of Nov. 20, 2020, Pfizer has requested the FDA for an Emergency Use Authorization (EUA) of its candidate to at-risk populations by late Dec. 2020.
Moderna is an American biotechnology company that develops drugs and vaccines using messenger RNA (mRNA). On Nov. 16, 2020, Moderna announced its preliminary Phase 3 trial data showed 94.5% effectiveness. This news strongly boosted its stock, which had suffered since the loss of a key patent battle in late July 2020. As of Nov. 17, 2020, Moderna’s COVID-19 candidate has been under the rolling review of European Medicines Agency (EMA). Additionally, Moderna has secured its distribution in the United Kingdom if the vaccine is approved. Even if Moderna’s vaccine is not widely distributed in the U.S., it seems Europe has put their trust in Moderna’s vaccine.
AstraZeneca is a multinational pharmaceutical company that specializes in the development of drugs for major diseases such as cancer, cardiovascular and respiratory issues. In its bid to provide a vaccine for COVID-19, Oxford University partnered with AstraZeneca to develop a vaccine. On Nov. 19, 2020, the results from its Phase 2 trial showed the vaccine to be safe as well as stimulate an immune response among all adults, including those over 70 years old. As promising as the results are, there was no measure of the vaccine’s efficacy in the Phase 2 trials. The Phase 3 trial is ongoing, and Oxford scientists hope to have the results by Christmas.
There appears to be light at the end of the long tunnel that the world has stumbled through since last November. With vaccine trials edging closer to completion and with distribution plans being laid out, the future looks promising for us all.