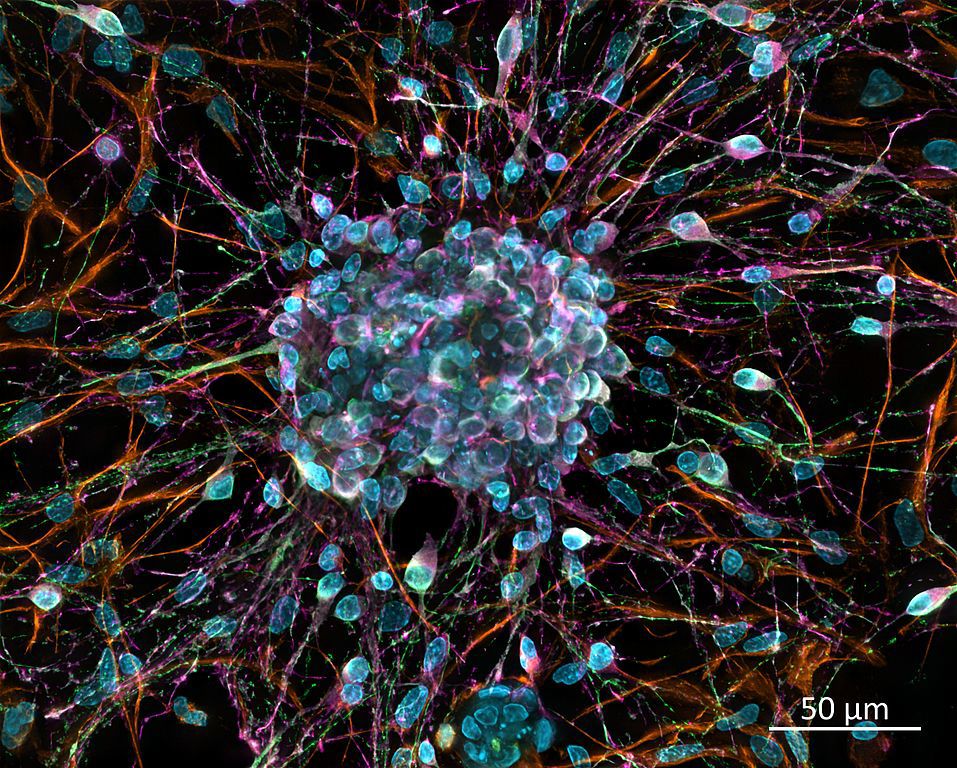
The youthful brain is a storm of activity. Information pours in through the senses while the brain builds fresh neurons to meet the need. But sometime within our first decade of life, the brain becomes content with its stock of neurons and the youthful bloom of new growth gives way to perpetual remodeling. Since neurons are among the longest-lived cells in our bodies, we usually don’t notice unless something goes wrong: a concussion, a stroke, or a hemorrhage. Without the capacity to generate new neurons, injuries to the brain can take a massive and irreversible toll—regions that were once awash with blinking neurons go dark forever. But what if we can turn the lights back on? What if our capacity for youthful neurogenesis wasn’t gone, but simply dormant? According to a 2019 study published in Molecular Therapy, adult mammalian brains can be convinced to build neurons again—they just need the right signal.
Despite getting most of the attention, neurons aren’t the only residents of the brain. Glial cells, while often overlooked, play a variety of supportive and restorative functions: they supply nutrients and oxygen to neurons, hunt down pathogens, and remove dead (or dying) neurons. Yu-Chen Chen and his colleagues at Pennsylvania State University focused on two crucial characteristics of neuroglia: first, adult mammalian brains never tire of generating new glial cells, and second, glial cells can transform into neurons. The conversion is rare and occurs predominantly during our early development, but it’s mediated by a regulatory protein we all possess, called NeuroD1. When this protein binds to the DNA of glial cells, it initiates their conversion into functional neurons.
In this study, the researchers used a highly targeted gene therapy to deliver NeuroD1 directly to the site of a brain injury. Rather than unloading NeuroD1 throughout the brain, they targeted a special subset of reactive glial cells that only arise in response to injury. To test the effectiveness of this treatment, the researchers exposed mice to a stroke-like injury and allowed them to recover on their own or with the NeuroD1 gene therapy. Mice treated with NeuroD1 regenerated ⅓ of the total neurons lost due to the injury and protected another ⅓ of the neurons that were injured, but not destroyed. Taken together, the treatment mice recovered ⅔ of the neurons that would’ve otherwise been lost. Further testing confirmed that the neurons created through this treatment were fully functional, including a behavioral analysis that showed significant improvements in both motor and cognitive function. Treatment with NeuroD1 may one day restore the youthful capacity for regeneration to adult brains.